Acidification
|
What is it? | 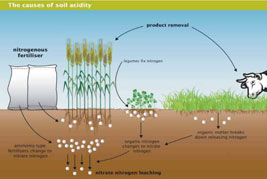 A diagrammatic representation of the causes of soil acidity showing the importance of product removal and nitrate leaching in the process |
Impact
Acidification of topsoils, and more seriously, subsoils will lead to lower yields, reduced pasture and crop options and contribute to wider catchment problems such as weed infestations, salinity and erosion.
In acidic soils, aluminium, iron and manganese can reach concentrations toxic to the roots and there may be deficiencies in molybdenum, boron, calcium, magnesium and potassium.
The impacts of soil acidity on agriculture and the wider community include:
- Increased nitrate contamination of groundwater and reduced water quality
- Reduced agricultural yields, farm income and domestic/export earnings
- Reduced options for agriculture (as only acid-tolerant plants can grow well in these conditions)
- Reduced vegetative cover, leading to accelerated run-off and erosion
- Irreversible clay structure damage (or hard setting)
- Declining pH of streams
- Increased infrastructure costs, and
- Decreased land values
Preventative measures:
- Monitor pH status of your paddocks (soil testing)
- Use forms of nitrogen fertilisers with a lower acidifying effect (Ammonium sulphate, MAP and DAP have ahigh acidifying potential)
- Feed out hay and silage on acidic paddocks
- Use better grazing and irrigation management
- Avoid irrigating when soil nitrate levels are highest ie. straight after grazing
- Growing deep-rooted perennial species that take up nitrogen from greater depth
- Applying lime, hence raising the pH
- Retain and sow deep-rooted perennial grasses (such as phalaris, cocksfoot) to reduce nitrate leaching
- Apply maintenance dressing of lime after the first remedial rate has been applied, especially after hay cutting
- Match fertiliser inputs to plant needs (to avoid excessive leaching)
- Use strategic grazing practices
- Use acid tolerant species (subterranean clover, perennial ryegrass, cocksfoot and native grasses)
DPI Information Note AG1182: Acid soils (PDF - 165 KB)
Impact of acid soils in Victoria
Measuring and interpreting soil pH
Soil Acidification in Australia (external link) on the Australian Natural Resources Atlas website


