Radiometrics
This information has been developed by Suzanne Haydon from the Geological Survey of Victoria, DPI.
What is radiometrics?
Radiometrics is a measure of the natural radiation in the earth’s surface, which can tell us about the distribution of certain soils and rocks. Geologists and geophysicists routinely use it as a geological mapping tool to tell them where certain rock types change. Radiometrics is also useful for the study of geomorphology and soils.
Radiometrics is also known as Gamma-Ray Spectrometry. A radiometric survey measures the spatial distribution of three radioactive elements (potassium-K, thorium-Th and uranium-U) in the top 30-45 cm of the earth’s crust. The abundances of K, Th and U are measured by detecting the gamma-rays produced during the natural radioactive decay of these elements.
What is radioactivity?
Radioactivity is the process where an unstable atom becomes stable through the process of decay, or breakdown, of its nucleus. During decay, energy is released in the form of three types of radiation; alpha, beta and gamma.
- Alpha radiation is helium nucleii, which are absorbed by a few cm of air;
- Beta radiation is electrons, which can travel up to a metre in air; and
- Gamma rays are parcels of electromagnetic radiation (similar to visible light).
How is radiometrics related to rock and soil type?
Radioactive elements occur naturally in the crystals of particular minerals. The abundance of minerals changes across the earth’s surface with variations in rock and soil type. Because the energy of gamma rays is related to the source radioactive element, they can be used to measure the abundance of those elements in an area. So by measuring the energy of gamma rays being emitted in an area, we can infer the presence of particular minerals in the earth’s surface.
How are gamma rays measured?
Gamma rays can be measured on the ground or from a low flying aircraft. The gamma rays are detected by a spectrometer, which counts the number of times each gamma ray of particular energy intersects it. The energy spectrum measured by the gamma ray spectrometer is in the range 0-3 MeV. Peaks in the spectrum can be attributed to potassium (K), thorium (Th) and uranium (U), the number of gamma ray counts across the whole spectrum are referred to as the total count (TC).
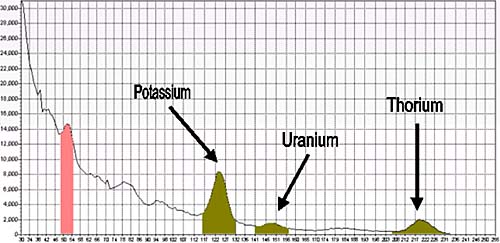
This spectrum has peaks corresponding to the presence of potassium, thorium and uranium. The heights of the peaks relate to the number of gamma rays recorded (in counts per second), and the horizontal axis relates to the energy of the gamma rays (high energy at left and low energy at right).
What are typical specifications for a regional airborne radiometric survey?
Regional airborne radiometric surveys in Victoria are conducted using either a helicopter or aeroplane. In mountainous areas a helicopter is used, flying at 80 m above the ground and following the terrain as closely as safety allows. The helicopter flies at speeds of 50 m/s (90 knots), recording a sample every 30-60 m along the flight path. Fixed wing aircraft are used where the terrain is flatter. The aeroplane flies at speeds of 70 m/s (130 knots), recording a sample every 50-80 m. Flight lines are usually 200 m apart in an east-west direction.
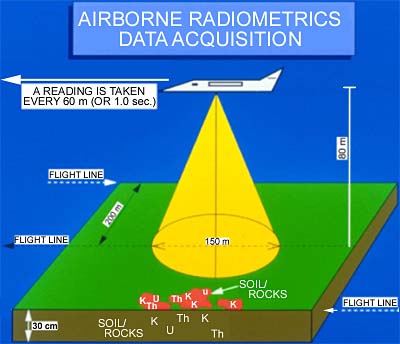
The field of view of a spectrometer flying at 80 m height is about 150 m, so there is overlap of samples along the flight line, and small gaps between samples on adjacent flight lines.
How are radiometric data displayed?
Radiometric data are commonly displayed as a map, with colours representing sample values. The figure below illustrates how values collected along flight lines are transformed into a coloured map with 100% coverage of the area.
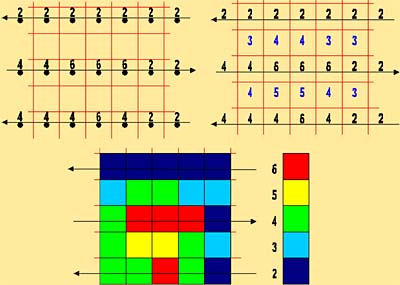
1. The data are collected as points along flight lines that are a certain distance apart.
2. A grid is applied to the area, and empty grid cells have their values calculated (interpolated) from the neighbouring cells.
3. Each grid cell is assigned a colour based on its cell value.
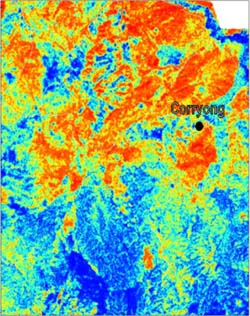
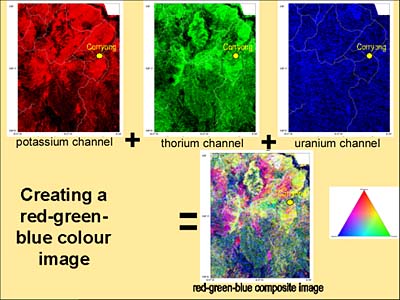
Below is an example of some real data from the Corryong area. The map shows potassium data; red areas have high gamma ray counts and the blue areas have low counts.
Another way to display radiometric data is to combine three datasets on the one picture using a red-green-blue ternary ratio. Each of the datasets are displayed using a different basic colour, which when combined make a colourful display with each shade representing different relative amounts of potassium, thorium and uranium. Usually the colours are displayed as follows:
Red = potassium
Green = thorium
Blue = uranium
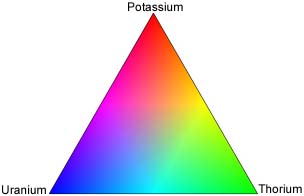
Using this colour scheme the following can be interpreted from the colours on the map:
Red = high potassium with low uranium and thorium
Blue = high uranium with low potassium and thorium
Green = high thorium with low potassium and uranium
Cyan = high thorium and uranium with low potassium
Magenta = high potassium and uranium with low thorium
Yellow = high potassium and thorium with low uranium
Black = low potassium, thorium and uranium
White = high potassium, thorium and uranium.
What are the units and what are typical values?
The units of measurement of a radiometric survey are counts per second. The values can vary depending on the survey height, type of spectrometer used and background radiation. To ensure that the units have geological significance and that adjacent surveys can be directly compared, the measurement units are converted to reflect mean-ground-level abundances of the radioelements.
Typical values are:
| Potassium | 0-450 cps | 0-5% potassium | percent |
| Thorium | 0-230 cps | 0-58 ppm equivalent thorium | parts per million |
| Uranium | 0-120 cps | 0-20 ppm equivalent uranium | parts per million |
| Total count | 0-4600 cps | 0-150 nGy/h air absorbed dose rate | nanoGray per hour |
Glossary of terms
Isotope: A chemical element whose atoms have a common number of protons and electrons, but the number of neutrons in their nucleus varies. Isotopes may be produced by nuclear reactions and the products are frequently radioactive. There are 92 naturally occurring elements, and 300 naturally occurring isotopes. Uranium has 92 protons and 142, 143 and 146 neutrons in isotopes U234, U235 and U238 respectively
Radiation: Energy that is transmitted, or radiated, in the form of rays, waves or particles eg. sound, heat or the electromagnetic spectrum (including light).
Gamma Ray: A form of electromagnetic radiation that is emitted by radioactive substances. The wavelength is 10-10 to 10-14 m, which is similar to, but shorter than the wavelength of X-rays. The energy of gamma rays varies from ten thousand (104) to ten million (107) electron volts (eV).
Nucleus, Nuclei: The centre of an atom. It comprises protons (with positive charge) and neutrons (which are electrically neutral). The nucleus of the hydrogen atom contains a single proton.
Atom: An atom is made up of a nucleus and its surrounding electrons. A hydrogen atom has its nucleus (containing a single proton) and a single electron. Chemical elements are composed of atoms.
Electromagnetic radiation: A wave of energy with wavelengths from 10-14 m to 104 m. This includes (in order of increasing wavelength): gamma rays, X-rays, visible light, microwaves and radio waves.
Spectrometer: An instrument that measures the abundance of gamma rays with different energy values.
Energy spectrum: Range of energy
MeV: eV = electron volt, a unit of energy. MeV= mega electron volt, or 106 eV. Therefore 1 MeV = 1000000 eV.
Grid: A pattern of regularly spaced vertical and horizontal lines.
Grid cell: A square or rectangle defined by the regularly spaced vertical and horizontal lines of a grid.
Radioelement: A naturally occurring or artificially produced radioactive element
Ternary ratio: A ratio of three variables
Radioactive decay: The process where an unstable "parent" element loses (emits) particles from its nucleus and becomes a stable "daughter" element.
Mineral: A naturally occurring inorganic substance with a characteristic chemical composition. Typically has a crystalline structure.
Crystal: A homogenous ordered solid with naturally formed plane faces and a limited chemical composition. Made up of atoms.


