Dispersion
Principal Binding Agents | Soil Texture | Dispersion | Slaking | Dispersion Animation
Dispersion describes the behaviour of clay particles separating from one other in a moist soil. Dispersion can cause soil aggregates to breakdown and the dispersed clay to clog soil pores. Structural decline and erosion usually result.
What causes dispersion?
To explain dispersion, it is easiest to begin with a simple animation of two dry aggregates of soil dispersing when placed in water.
Clays particles are small in size (less than 0.002 mm) but have a very large surface area. The surface area of all clays is negatively charged. This is because of the complex arrangement of elements (e.g. aluminium, oxygen, silicon) that make up the clay structure. Positive ions (cations such as calcium, Ca2+) present in the soil are electrostatically attracted to the negative clay surface and neutralise the charge in the clay. As all the negative charges on the clay are neutralised, a layer of positive charge surrounds the clay particle. This layer of positive charge is also known as a 'shell'.
The width of the shell depends on whether the cations are single (sodium, Na+), double (calcium, Ca2+) or triple (aluminium, Al3+) charged. That is, one Na+ will neutralise one negative charge on the clay, whereas one Al3+ will neutralise three negative charges on the clay.
Cations floating around in the soil solution as salts, also affect the width of the shell. Cations 'attached' to the clay particle diffuse away from the surface of the clay until the concentration of cations is equal to the concentration of cations in the soil solution. Thus, the saltier the soil solution, the thinner the layer of positive charge surrounding the clay particle.
| Like charges repel one another, however, this can be overcome by close distance nuclear attraction, called Van der Waal's forces. If the shell is thick, the clay particles are going to have trouble coming close enough together for the Van der Waals' forces to act and for the particles to flocculate. They will tend to remain as separate (colloidal) entities – and the clay will be dispersed. | 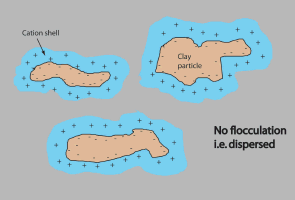 No-flocculated soil sample |
| If the shell is thin, the particles can come close together for attractive Van der Waal's forces to take hold and for the clay particles to flocculate. | 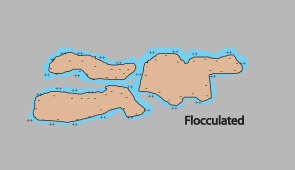 Flocculated soil sample |
Sodic soils are typically highly dispersive for the reasons outline above. Sodic soils have a high concentration of exchangeable Na+, therefore much of the negative charge on the clay is neutralised by Na+, creating a thick layer of positive charge that may prevent clay particles from flocculating.
The cations ‘attached’ to the clay particle are exchangeable with other cations in the soil solution. Which cations ‘attach’ to the clay depends on the concentration and physical size of the cations.
What dispersion looks like
The following two videos illustrate dispersion when susceptible soil aggregates are immersed in water.
In the first video dispersion is preceded by slaking, in the second video, slaking is insignificant
A demonstration of clay dispersion, preceded by slaking
The video shows two aggregates of soil immersed in water. Both aggregates slake on immersion. However, as time progresses the right hand aggregate disperses completely and all that remains at the 30 minute mark are a few sand grains and a cloud of clay.
A demonstration of clay dispersion, with only minor slaking
The video shows an aggregate of soil resisting slaking (although there is some release of air bubbles) and undergoing visual clay dispersion within 30 seconds of immersion.
Effect of dispersion
Dispersive clays are relatively mobile within a soil. As they move they tend to clog soil pores and form layers of low permeability. Surface sealing, restricted water transport and drainage, reduced macro-porosity, increased swelling, and increased soil erosion are likely outcomes.
Effect of flocculation
Flocculated clays tend to be stable and hence result in a relatively resilient soil structure. Floccualated clays encourage soils to aggregate, and soils with stable aggregates allow water infiltration and drainage, provide pore spaces for air and water storage, provide housing for soil biology, and are more resistant to erosion.
Treating dispersive soils
If you have a dispersive soil, the first step is to find out if you soil is dispersive throughout the soil profile. If your soil is sodic, soil testing to find out the concentration of exchangeable and soluble salts in your soil is needed. These numbers will provide you with the information to calculate whether treating the soil with gypsum is practical and cost effective for you.
Increasing the organic matter content of your soils will also significantly improve the stability of your soil and its ability to resist dispersion when the soil is moist.
Lastly, protecting the surface of your soil by maintaining ground cover will decrease the impact of raindrops and other mechanical disturbances on your soil that breakdown the soil and cause dispersion.
Assessment of dispersion
To assess your soil to see if it will disperse, there is a relatively quick and simply test that you can do yourself at home.
| Practical Note: Aggregate Stability Well aggregated soil is important. It has pores between aggregates and within the aggregate. Large pores allow for the exchange of oxygen and other gases with the atmosphere, while small pores hold plant available water and dissolved nutrients. |  |
| Quick Reference Guide: Assessing aggregate stability When a fragment of soil is immersed in fresh water, there are four things that can happen:
|  |
Treating dispersive soils
If you have a dispersive soil, the first step is to find out if you soil is dispersive throughout the soil profile. If your soil is sodic, soil testing to find out the concentration of exchangeable and soluble salts in your soil is needed. These numbers will provide you with the information to calculate whether treating the soil with gypsum is practicable and cost effective for you.
Increasing the organic matter content of your soils will also significantly improve the stability of your soil and its ability to resist dispersion when the soil is moist.
Lastly, protecting the surface of your soil by maintaining ground cover will decrease the impact of raindrops and other mechanical disturbances on your soil that breakdown the soil and cause dispersion.
Further information
More technical information on aggregate slaking and clay dispersion is available on VRO under ‘soil management’.


